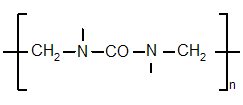Urea-formaldehyde
Urea-formaldehyde, also known as urea-methanal, named so for its common synthesis pathway and overall structure,[1] is a non-transparent thermosetting resin or plastic, made from urea and formaldehyde heated in the presence of a mild base such as ammonia or pyridine. These resins are used in adhesives, finishes, MDF, and molded objects.
Contents |
[edit] Properties
Urea-formaldehyde resin's attributes include high tensile strength, flexural modulus, and heat distortion temperature, low water absorption, mould shrinkage, high surface hardness, elongation at break, and volume resistance.
[edit] Chemical structure
The chemical structure of UF resins can be described as that of polymethylene. This description leaves the details of the structure undetermined, which can vary linearly and branched. These are grouped by their average molar mass and the content of different functional groups. Changing synthesis conditions of the resins give good designing possibilities for the structure and resin properties.
[edit] Production
There is approximately 1 million metric tons of urea-formaldehyde produced every year. Over 70% of this production is then put into use by the forest industry products. It produces a great resin for bonding particleboard (61%), medium density fiberboard (27%), hardwood plywood (5%), and laminating adhesive (7%).
[edit] General uses
Urea-Formaldehyde is everywhere and used in many manufacturing processes due to its useful properties. Examples include decorative laminates, textiles, paper, foundry sand molds, wrinkle resistant fabrics, cotton blends, rayon, corduroy, etc. It is also used to glue wood together. Urea formaldehyde was commonly used when producing electrical appliances casing (e.g. desk lamps).
The product is widely chosen as an adhesive resin due to its high reactivity, good performance, and low price. Urea-formaldehyde resin is a chemical combination of urea and formaldehyde. Amino resins are considered a class of thermosetting resins of which urea-formaldehyde resins make up 80% produced globally. Examples of amino resins include automobile tires in order to improve the bonding of rubber to tire cord, paper for improving tear strength, molding electrical devices, molding jar caps, etc.
[edit] Agricultural use
Urea formaldehyde is also used in agriculture as a controlled release source of nitrogen fertilizer. Urea formaldehyde’s rate of decomposition into CO2 and NH3 is determined by the action of microbes found naturally in most soils. The activity of these microbes, and, therefore, the rate of nitrogen release, is temperature dependent. The optimum temperature for microbe activity is approximately 70-90 °F (approx 20-30°C).
[edit] Urea-formaldehyde foam insulation
Urea-formaldehyde foam insulation (UFFI) can make a great synthetic insulation. It is basically foam like shaving cream and easily injected into walls with a hose. It is made by using a pump set and hose with a mixing gun to mix the foaming agent and resin. The liquid foam is sprayed into areas in need of insulation. It hardens within minutes but cures within a week. UFFI is generally spotted in homes built before the seventies; one should look in basements, crawl spaces, attics, and unfinished attics. Visually it looks like oozing liquid that has been hardened. Over time, it tends to vary in shades of butterscotch but new UFFI is a light yellow color. Early forms of UFFI tended to shrink significantly though updated catalysts and foaming technology have reduced shrinkage to minimal levels (between 2-4%). The foam dries with a dull matte color with no shine. It will be a dry and crumbly texture.
[edit] Removal
Removal is a very expensive process, and the UFFI must be handled with extreme caution and care.[citation needed] The affected walls must be opened in order to remove the foam. The worker then neutralizes the wall cavity with sodium bisulphite solution[citation needed]. Then the original drywall or plaster must be replaced. Official protocols must be followed for the removal and disposal[citation needed]. Qualified personnel need a permit to even begin the process[citation needed]. UFFI can be found in random places as well because it was installed under great air pressure of up to 100 pounds per square inch[citation needed].
[edit] Safety concerns
Urea-formaldehyde foam insulation (UFFI) started being used in the 1970s. Homeowners used UFFI as a wall cavity filler at the time in order to conserve energy. In the 1980s, concerns began to develop about the toxic formaldehyde vapor emitted in the curing process, as well as from the breakdown of old foam. Emission rates of more than 0.1 parts per million (ppm) takes a toxic toll on humans. Consequently, its use was discontinued. The urea-formaldehyde emissions decline over time and significant levels should no longer be present in the homes today.[2] Modern replacement options include melamine formaldehyde resin and polyurethane.
UFFI was usually mixed at the location of use while constructing the home’s walls. It was than injected inside the walls, the curing process occurs, and the final product acts as an insulating agent. Historically, less information was known about the toxic health effects of formaldehyde, sometimes extra formaldehyde was added to the mixture to guarantee that the curing process would occur completely. UFFI is not a well sealed product; it is put into place and left there in the open. It is dangerous when wet or exposed because this is when levels of formaldehyde can be released into the home. UFFI has an unfortunate direct link to temperature, they are positively correlated. When temperature rises, the levels of formaldehyde that leaks will also increase. Solutions for positive tests (testing for formaldehyde emissions in the air) of UFFI leakage is to seal off the outlet for the vapors. One would seal all the cracks and apply multiple layers of vapor barrier paint, and then further covering this with mylar of vinyl paper. Aluminum foil is a useful alternative for barricading vapors. Removal is a costly and tedious option for UFFI and would require a replacement insulation installation.
[edit] Health effects
Health effects of Urea-Formaldehyde are put into effect when the elevated level is reached.[clarification needed] Urea-formaldehyde releases formaldehyde emissions into the air. This triggers watery eyes, nose irritations, wheezing and coughing, fatigue, skin rash, severe allergic reactions, burning sensations in the eyes and throat, nausea, and difficulty in breathing in some humans (usually 0.1 ppm). Studies have shown that exposure may cause cancer in humans and animals. Occupants of UFFI insulated homes experienced systemic symptoms such as headache, malaise, insomnia, anorexia, and loss of libido. Irritation of the mucus membrane (specifically eyes, nose, and throat) is a common upper respiratory tract symptom to be found in both home and work environments containing enough UFFI- insulation concentration. However when compared to control groups, the frequency of symptoms did not exceed the controls expect when it came to wheezing, difficult breathing, and a burning skin sensation. Controlled studies have suggested that tolerance to formaldehydes odor and irritating effects can occur over a prolonged exposure. Tolerance, sensitivity, and idiosyncratic reactions should be considered for further investigation. The effects of cancer or congenital malformations may be too low to assess.
[edit] See also
[edit] References
- Urea formaldehyde (Plastics Historical Society)
- Urea-Formaldehyde Foam Insulation(Canada Mortgage and Housing Corporation)
- Indoor Air Quality: Formaldehyde(US Environmental Protection Agency)
- UFFI (UK Health and Safety Executive)
- (United States Environmental Protection Agency)
- (Environmental and Occupational Health Assessment Program|Connecticut Department of Public Health)
- (Consumer Product Safety Commission|Consumer Product Safety Commission)
- (Forest Products Laboratory: USDA Forest Service)
- [Dunky, M., “Urea-formaldehyde (UF) adhesive resins for wood,” International Journal of Adhesion and Adhesives, 1998. (18:2).]
- (Encyclopæia Britannica)
- (PropEx.com)
- (U.S. Dept. of Labor, Occupational Safety and Health Administration (OSHA))