Car Battery
What should you look for in a car battery? Here are further guidelines. |
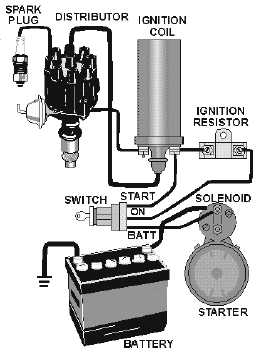
| Why won't my engine start? |
|
Battery Details Battery Life Battery Testing Battery Usage Rechargeable Batteries Non-Rechargeable Batteries |
|
What is a Battery? Where are batteries used? What are the types of batteries? What materials are required to manufacture a battery? How is a battery made? What is the external case of a battery made of, high-density tough polypropylene or hard rubber? How do you determine the dimensions of the external case of a battery? What type of material is it? Why doesn't it get damaged by an electrolyte or current? What types of materials are used in a battery? Where is lead used in a battery? Where is lithium used in a battery? What others materials can be used in their place? How are lead and lithium extracted? What are battery plates? How are plates of a battery made? Why do we need them? What type of electrolyte is in a battery? What should be the electrolyte level in a battery? How does a battery work? How do batteries die? Why are vehicles negatively grounded? How do you do capacity testing of a battery? What is a state-of-charge and how do you do it? What is a battery hydrometer? What are the uses of a battery hydrometer? How do you use a battery hydrometer? What is a low electrolyte level? How and when can a battery explode on its own? What precautions should one use while handling a battery? What type of cables should one use for a battery? What is an alternator belt? What should you look for and ask during inspection? When should you refill electrolytes? When should you recharge a battery? Why use Lithium batteries? Why use Rechargeable Lithium Ion batteries? Why use Rechargeable NiCd batteries? Why use Rechargeable NiMH batteries? Why use alkaline batteries? Why use Zinc Air batteries? Why use Silver Oxide batteries? Why use heavy duty batteries? What is an Ampere? What is mAh? What is Ah? What is Cut off voltage? What does OEM mean? What is a Cycle? What is a Deep Cycle battery? Why use an AGM battery? What is MCA? What is CCA? What is PCA? What is a Volt? What is a Watt? What is an OHM? What is RC? What is a Starting battery? What is sulfation of batteries? Why use Gel batteries? What is a "smart" and "dumb" battery? What is self-discharge? What is Shelf life? What is a Capacity and why should I care? How do I select a charger for my SLA battery? How do I find the right battery for my cell phone? How do I connect batteries in series? How do I jump start my battery using booster cables? How do I connect batteries in parallel? What do I do if I cant find the battery I am looking for? Can you build custom batteries? Performance How long should a rechargeable last? Does it help to store batteries in refrigerator? My new battery isn't charging is it defective? What is the battery standby and talk time? Do batteries self discharge not in use? Do I ever need to add acid to my battery? Can batteries freeze? I just received my new battery. Why isn't it working? Will my device's performance differ if I use your aftermarket battery? How can I maximize performance? Is it possible to upgrade the battery in my device to a newer chemistry? Can I overcharge a battery? Disposal/Recycling How do I dispose of batteries? Are lead acid batteries recycleable? |