|
How many million barrels of fuel oil is the government required to release from the emergency stockpile? See what was circulated by the government on November 23. Here are further guidelines. |
2. Energy equipment
|
What is on the list of energy equipment for professionals in the government? Here are further guidelines. |
3. Energy-related human resources
|
Which professionals are the most important for energy-related human resources of the government? Here are further guidelines. |
4. Government energy departments
|
Who must participate in government energy departments’ global deliberations? Here are further guidelines. |
5. Government energy reforms within public services
|
Government energy reforms within public services: What needs to be accomplished to improve government services? Here are further guidelines. |
6. Education about government energy resources in schools, colleges, and universities
|
What education about government services needs to be provided? Here are further guidelines. |
7. Government energy regulations
|
Government energy regulations: What has been elaborated?
What must everyone be updated on relevant to energy regulations on or after October 11, 2021? These are all government resources for public services. Do not try to monopolize or sideline government energy services. Petroleum products have to be categorized relevant to public services. Petroleum products for essential services. Petroleum products for recreational activities. Petroleum products for essential services: How must the regulations proceed on or after November 27, 2021? Here are various examples. Issue number 1. Food stuffs have to be placed in the community so that residents can consume a balanced diet. Trucks have to be mobilized to bring the essential products that need fuel oil. The government’s provision of free fuel oil for these trucks is justified to bring a balanced diet for residents. What did you understand? Issue number 2. On November 23, 2021, this was circulated. 50 million Americans within a week will be using private vehicles that need fuel to travel around for the holidays. This is not an essential service. If fuel oil for these services is expensive, it is not a problem. Petrol pumps On November 25, 2021, in one region the dealer margin was 6 percent: Why not make this a public service? In one region a shutdown of petrol pumps was circulated after 6 AM on November 25, 2021. How about placing government officers at petrol pumps compared to petroleum dealers? Here are further guidelines. |
8. Energy product- or service-specific issues of government
|
What energy product- or service-specific issues of the government need to be improved? Here are further guidelines. |
9. Corruption monitoring in public services
|
Who has what responsibilities in the government department of energy? Who has engaged in corruption in the government department of energy and sabotaged government services? Here are further guidelines. |
10. Issues relevant to energy that are occasionally relevant to government
|
Are there any energy issues in your area relevant to government services? What are the issues? |
|
1. Energy gives us light. 2. Energy gives us heat. 3. Energy makes things move. 4. Energy makes things grow. 5. Energy makes technology work. What is Energy? What is Renewable Energy? What are renewable energy sources? How does Renewable Energy affect the supply of electricity? Why use Renewable Energy? How much Renewable Energy do we have? Where does your electricity come from? | ||||||||||||||
Energy Sources
|
| Diesel |
| Petrol |
| K.oil |
LPG
How many total LPG outlets are in the state as of March 30, 2011? |
| Exploration |
| Production |
| Refining |
| International operations |
| Organic chemistry |
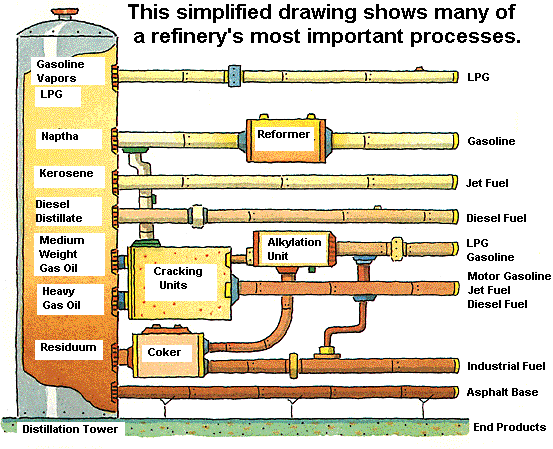 |
| Oil Refineries |
