|
What is Protein?
A protein is a long train of amino acids linked together. Proteins have different functions; they can provide structure (ligaments, fingernails, hair), help in digestion (stomach enzymes), aid in movement (muscles), and play a part in our ability to see (the lens of our eyes is pure crytalline protein). Protein is a long chain molecule made up of amino acids joined by peptide bonds. Protein forms the structural material of bodily tissues. Proteins, the principal constituents of the protoplasm of all cells, are of high molecular weight and consist essentially of combinations of a amino acids in peptide linkages. Twenty different amino acids are commonly found in proteins and each protein has a unique, genetically defined amino acid sequence which determines its specific shape and function. They serve as enzymes, structural elements, hormones, immunoglobulins, etc. And are involved in oxygen transport, muscle contraction, electron transport and other activities throughout the body and in photosynthesis. Origin: Gr. Protos = first The most important function of protein is to build up, keep up, and replace the tissues in your body. Your muscles, your organs, and some of your hormones are made up mostly of protein. Protein also makes antibodies and hemoglobin (responsible for delivering oxygen to your blood cells). Our body is able to produce 14 of the 20 amino acids. We have to get the remaining amino acids from the foods we eat. fish. But proteins are complex and have many different functions within the human body. Proteins form the major components of muscles, skin, tendons, blood vessels, hair, and cores of bones and teeth. They help you grow, heal wounds, and make up collagen - the connective tissue that gives your body its shape. Other proteins, called enzymes, help generate energy in your body. Proteins called hormones act as internal “project managers”, ensuring your body runs itself properly. Insulin and glucagons, for example, are the hormones that control blood sugar levels. And proteins called antibodies are important components of your immune system, warding off foreign particles like bacteria. Without protein, life would be impossible. Practically every cell in your body spends considerable time and energy manufacturing various kinds of proteins. Every imaginable part and function of your body has protein involved in some way, from the enzymes that are critical to the digestion of foods to the fibers that plug leaky blood vessels. But before you get carried away with putting protein on a nutritional pedestal, it can also be said that life would be impossible without sugars (carbohydrates) and fats (lipids). Protein is simply a name for a nutritional family. We could just as easily have said this is a photo of the Jones or Smith family. To pick out their distinctive family characteristics we need to look closer. Do they all have large noses, blue eyes, and freckles? Just as human families have one trait in common with all othersthey are all members of the human speciesso do proteins. The principal trait for all proteins, large or small, is that they are composed of amino acids. With about 20 different kinds available, every cell of your body can choose from the pool of amino acids to construct specific proteins to meet very diverse needs of human physiology. For example, there are boats to shuttle oxygen around (hemoglobin), taxis for small fats (LDL), and ballistic missiles to destroy invaders (immunoglobulins). Put two amino acids together and you get a dipeptide (di = "two"); put three amino acids together and the result is a tripeptide (tri = "three"); finally, a complex construction of many amino acids makes a polypeptide (poly = "many"). Human insulin, for instance, which is responsible for getting glucose into the muscles, is composed of 51 amino acids in two short polypeptide chains. As the digestive process kicks into high gear, "intelligent" receptors on the large intestinal surfaces have to decide which amino acid, dipeptide, or tripeptide to absorb, and which will have to wait. This competition among the amino acids for absorption is not a big deal, since the body's receptors "know" which amino acids are needed most of the time. However, if your intestinal tract is flooded with amino acid supplements, the absorptive cells cannot deal with these refined and readily available amino acids all at once. Inevitably, the "pushier" amino acids, or the ones in greatest concentration, leave the receptors no choice but to take them in first. The constant construction of new proteins, along with the destruction and elimination of old proteins, means that a continual supply of amino acids must be made available via nutrition to maintain a healthy balance. Different foods provide essential amino acids in varying proportions. We now have clear evidence that even if a diet is derived exclusively from the vegetable kingdom, it can provide all the necessary amino acids for optimal health. It is generally sufficient simply to have a consistent supply of a variety of vegetables, legumes, and grains throughout the week. Protein is one of the three macronutrients commonly identified as a dietary requirement. It represents nitrogen-containing compounds for which amino acids are the basic structural units. Amino acids are small organic compounds containing at least one amino group and an organic acid group. The differences between amino acids lies in the differences between the amino acid side proteins are the most abundant organic compound of the body. More than fat, usually. Much more than carbohydrate. About 65% of the total body protein lies in the skeletal muscles. Proteins function primarily in the growth and repair of body tissue. Just about every cell in our body has a protein component, and we are unable to synthesize new cells without the requisite building blocks. Hair, nails, skin contain protein. Blood contains plasma proteins; hemoglobin has a protein component. Proteins are components of some antibodies. Many hormones are proteins (like insulin). In fact, the protein content of the average cell is 16% of its total mass. There are more than 50,000 different proteins in our bodies. These are all made from about 22 different amino acids. Our bodies can synthesize 14 of these 22 amino acids, we cannot make 8 of them, and these 8 must come from food. These 8 are called the essential amino acids. Sometimes we cannot synthesize other amino acids and therefore they too must come from diet. Protein is a large, complex molecule composed of amino acids. The sequence of the amino acids, and thus the function of the protein, is determined by the sequence of the base pairs in the gene that encodes it. Proteins are essential to the structure, function, and regulation of the body. Examples are hormones, enzymes, and antibodies. Many bodybuilders use Protein to help build muscle in the body. A protein is a complex, high molecular weight organic compound that consists of amino acids joined by peptide bonds. Proteins are essential to the structure and function of all living cells and viruses. Many proteins are enzymes or subunits of enzymes. Other proteins play structural or mechanical roles, such as those that form the struts and joints of the cytoskeleton. Still more functions filled by proteins include immune response and the storage and transport of various ligands. In nutrition, proteins serve as the source of amino acids for organisms that do not synthesize those amino acids natively. Proteins are one of the classes of bio-macromolecules, alongside polysaccharides and nucleic acids, that make up the primary constituents of living things. They are amongst the most actively studied molecule in biochemistry and were discovered by Jöns Jacob Berzelius, in 1838. Proteins are amino acid chains, made up from 20 different L-α-amino acids, also referred to as residues, that fold into unique three-dimensional protein structures. The shape into a which a protein naturally folds is known as its native state, which is determined by its sequence of amino acids. Below about 40 residues the term peptide is frequently used. A certain number of residues is necessary to perform a particular biochemical function, and around 40-50 residues appears to be the lower limit for a functional domain size. Protein sizes range from this lower limit to several hundred residues in multi-functional proteins. Very large aggregates can be formed from protein subunits, for example many thousand actin molecules assemble into a an actin filament. Large protein complexes with RNA are found in the ribosome particles, which are in fact 'ribozymes'. Biochemists refer to four distinct aspects of a protein's structure: Primary structure: the amino acid sequence Secondary structure: highly patterned sub-structures--alpha helix and beta sheet--or segments of chain that assume no stable shape. Secondary structures are locally defined, meaning that there can be many different secondary motifs present in one single protein molecule Tertiary structure: the overall shape of a single protein molecule; the spatial relationship of the secondary structural motifs to one another Quaternary structure: the shape or structure that results from the union of more than one protein molecule, usually called subunit proteins subunits in this context, which function as part of the larger assembly or protein complex. In addition to these levels of structure, proteins may shift between several similar structures in performing of their biological function. In the context of these functional rearrangements, these tertiary or quaternary structures are usually referred to as "conformations," and transitions between them are called conformational changes. The primary structure is held together by covalent peptide bonds, which are made during the process of translation. The secondary structures are held together by hydrogen bonds. The tertiary structure is held together primarily by hydrophobic interactions but hydrogen bonds, ionic interactions, and disulfide bonds are usually involved too. The two ends of the amino acid chain are referred to as the carboxy terminus (C-terminus) and the amino terminus (N-terminus) based on the nature of the free group on each extremity. Two amino acids are combined in a condensation reaction. Notice that the peptide bond is in fact planar due to the delocalization of the electrons. The sequence of the different amino acids is considered the primary structure of the peptide or protein. Counting of residues always starts at the N-terminal end (NH2-group). Proteins are involved in practically every function performed by a cell, including regulation of cellular functions such as signal transduction and metabolism. For example, protein catabolism requires only a few enzymes termed proteases. Various molecules and ions are able to bind to specific sites on proteins. These sites are called binding sites. They exhibit chemical specificity. The particle that binds is called a ligand. The strength of ligand-protein binding is a property of the binding site known as affinity. Since proteins are involved in practically every function performed by a cell, the mechanisms for controlling these functions therefore depend on controlling protein activity. Regulation can involve a protein's shape or concentration. Some forms of regulation include: Allosteric modulation: When the binding of a ligand at one site on a protein affects the binding of ligand at another site. Covalent modulation: When the covalent modification of a protein affects the binding of a ligand or some other aspect of a the protein's function. Proteins are generally large molecules, having molecular masses of up to 3,000,000 (the muscle protein titin has a single amino acid chain 27,000 subunits long). Such long chains of amino acids are almost universally referred to as proteins, but shorter strings of amino acids are referred to as "polypeptides," "peptides" or very rarely "oligopeptides". The dividing line is somewhat undefined, although a polypeptide may be less likely to have tertiary structure and may be more likely to act as a hormone (like insulin) rather than as an enzyme or structural element. Proteins Quiz - College Level Questions Question 1 : How many different kinds of proteins does a typical cell contain? A. 100 B. 1,000 C. 10,000 D. 100,000 Question 2 : What have scientists already learned from studying the detailed, three-dimensional structures of proteins? A. What causes certain diseases B. How to design drugs C. How viruses infect cells D. How photosynthesis works E. All of the above Question 3 : Which of the following are involved in X-ray crystallography? A. X-ray beams B. Microscopic crystals C. Hard work and luck D. High-powered computers E. All of the above Question 4 : Which of the following is not true about nuclear magnetic resonance (NMR) spectroscopy? A. The magnets used in NMR spectroscopy are thousands of times stronger than the magnetic field on Earth's surface B. NMR uses crystals in a solution C. NMR magnets are superconductors D. NMR uses radio wave pulses Question 5 : What do the areas in red represent? 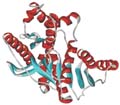 A. The helical structure of DNA B. Corkscrews C. The RNA part of the ribosome D. Alpha helices, which are organized sections of proteins Proteins Quiz - College Level Answers Question 1 : How many different kinds of proteins does a typical cell contain? Answer C. 10,000 Question 2 : What have scientists already learned from studying the detailed, three-dimensional structures of proteins? Answer E. All of the above Question 3 : Which of the following are involved in X-ray crystallography? Answer E. All of the above Question 4 : Which of the following is not true about nuclear magnetic resonance (NMR) spectroscopy? Answer B. NMR uses crystals in a solution Question 5 : What do the areas in red represent? Answer D. Alpha helices, which are organized sections of proteins Proteins Quiz - Graduate Level Questions Question 1 : Which of the following can cause disease? A. A protein that contains an incorrect amino acid B. A protein that is incorrectly folded C. A protein that is missing from the body D. All of the above Question 2 : What is structure-based drug design? A. A rigorous, highly structured way to create new medicines B. A strategy that relies on the structure of the drug delivery method (tablet, time-release capsule, syringe, nasal spray, etc.) C. A method of designing medicines that relies on computational models of protein structures D. All of the above Question 3 : Which of the follow statements are true about graphic representations of proteins (computer models)? A. Ribbon diagrams highlight organized regions of the proteins B. Space-filling molecular models attempt to show atoms as spheres whose size correlates with the amount of space the atoms occupy C. A surface rendering of the protein shows its overall shape and surface properties D. All of the above Question 4 : Why do X-ray crystallographers like to use synchrotrons? A. Synchotrons generate intense beams of X-rays that can be tuned to the appropriate wavelength B. Synchrotrons analyze and refine structural data, so scientists don't need computers to do this C. The X-rays from synchrotrons are much less harmful than traditional X-ray sources D. All of the above Question 5 : Why does NMR have an advantage over X-ray crystallography for certain research? A. It can determine the structures of proteins that are too large for X-ray crystallography B. Proteins are closer to their natural state in NMR studies, which rely on a solution, than they are in the crystalline form used for X-ray crystallography C. It is much faster than X-ray crystallography D. It doesn't require the use of isotopes E. It shows hydrogen atoms, which do not appear in X-ray crystal structures Proteins Quiz - Graduate Level Answers Question 1 : Which of the following can cause disease? Answer D. All of the above Question 2 : What is structure-based drug design? Answer C. A method of designing medicines that relies on computational models of protein structures Question 3 : Which of the follow statements are true about graphic representations of proteins (computer models)? Answer D. All of the above Question 4 : Why do X-ray crystallographers like to use synchrotrons? Answer A. Synchotrons generate intense beams of X-rays that can be tuned to the appropriate wavelength Question 5 : Why does NMR have an advantage over X-ray crystallography for certain research? Answer B. Proteins are closer to their natural state in NMR studies, which rely on a solution, than they are in the crystalline form used for X-ray crystallography Proteins Quiz - High School Level Questions You can study the class notes before you take the quiz. Question 1 : Proteins are long chains of . . . A. nucleotides B. sugar and spice and everything nice C. amino acids D. fatty acids E. monosaccharides Question 2 : Proteins do all of the following things in the body except which one of the following? A. Carry genetic information B. Speed up chemical reactions C. Digest food D. Carry oxygen in blood E. Defend against microorganisms Question 3 : Proteins are made in molecular factories called . . . A. mitochondria B. ribosomes C. synchrotrons D. vesicles Question 4 : Which is the most commonly used technique to determine protein structures? A. Nuclear magnetic resonance (NMR) spectroscopy B. Computer modeling C. X-ray crystallography D. Magnetic resonance imaging (MRI) E. Kryptonic X-ray vision Question 5 : Which of the following protein structures is most likely to act like a strong fiber in the body? Proteins Quiz - High School Level Answers Question 1 : Proteins are long chains of . . . Answer C. amino acids Question 2 : Proteins do all of the following things in the body except which one of the following? Answer A. Carry genetic information Question 3 : Proteins are made in molecular factories called . . . Answer B. ribosomes Question 4 : Which is the most commonly used technique to determine protein structures? Answer C. X-ray crystallography |
