Elements, Compounds and Mixtures
Solid, Liquid and Gas.
|
Elements. Compounds. Mixtures. Mixtures - Separation Techniques - Filtration. Mixtures - Separation Techniques - Crystallisation. Mixtures - Separation Techniques - Fractional Distillation. Mixtures - Separation Techniques - Chromatography. Solid, Liquid and Gas. Solid, Liquid and Gas - Particle Motion. Solid, Liquid and Gas - Structure and Properties. Solid, Liquid and Gas - Melting and Boiling. Brownian Motion. Diffusion. Diffusion of Bromine through Air. Diffusion of Hydrogen Chloride and Ammonia. Chemical and Physical Properties. Hazard Symbols. Questions. Elements An element is defined as a pure substance made up of one type of atoms and cannot be further subdivided into simpler substances by any physical or means. An element can be represented by using a symbol. Examples of Elements The symbols H2 and Fe represent the elements Hydrogen and Iron respectively. An element is a substance made from only one type of atom. Carbon is made entirely from carbon atoms. Sodium is made entirely from sodium atoms. An element cannot be broken down (chemically) into a more simple substance. Atoms of the same element have the same atomic number. Elements are the simplest pure substance. An element can not be changed into a simpler substance by heating or any chemical process. The smallest particle of an element that has the properties of that element is called an atom. An atom is the basic building block of matter. There are more than one hundred known elements in the universe listed on the periodic table of elements. These elements combine in such a way to create millions of compounds. Elements All elements are made of atoms. Atoms of the same element are alike. Atoms of different elements are different. In 1813, a system of representing elements with symbols was introduced. Each symbol consists of one or two letters. Two letters are needed for a chemical symbol when the first letter of that element’s name has already been used. Compounds. A compound is a substance made from two or more elements which have reacted chemically with each other. A compound is a completely new material which will often have totally different properties from the elements which made it. Compounds are also pure substances. But compounds are made from more than one element. Water is a compound. Water can be broken down into simpler substances – hydrogen and oxygen. For example The element sodium is a highly reactive metal. The element chlorine is a yellow-green poisonous gas (non-metal). When the two react together, they form a compound called sodium chloride. Sodium chloride is common salt, which you eat with food. You wouldn't want to eat the elements! You cannot separate the elements of a compound by physical methods. It can only be done by using more chemical reactions or by passing electricity through it (if it conducts electricity). A compound is defined as a pure substance made up of two or more types of elements (atoms) chemically combined in a fixed proportion, and it can be further subdivided into simpler substances by chemical means only. A molecule is the smallest part of a compound, whose properties are the same as those of the compound. A compound can be represented by using a chemical formula. Examples of Compounds The chemical formulae H2O and FeS represent the compounds water and Ferrous sulfide (Iron [I] sulfide) respectively. Mixtures A mixture is defined as an impure substance made up of two or more types of elements (atoms) or compounds or both mechanically mixed in any proportion, and it can be further subdivided into simpler substances by physical (mechanical) means. The constituents of a mixture retain their original properties. The constituents of a homogenous mixture are uniformly mixed thoroughout the mixture. The properties and composition of a homogenous mixture are the same throughout the mixture. The constituents of a heterogenous mixture are not uniformly mixed thoroughout the mixture. The properties and composition of a heterogenous mixture are not the same throughout the mixture. Examples of Mixtures Stainless steel is a mixture (alloy) of iron, carbon, chromium, and nickel. Carbon gives hardness to the mixture. Chromium and nickel give a silvery look to the mixture. Potassium sulfide solution is a homogenous mixture. A mixture of water and oil is heterogenous in nature. A mixture contains two or more substances which have not reacted chemically with each other. A mixture is made of little bits of each substance mixed together. A mixture can be separated by physical methods, a compound can not. For example A mixture of iron filings and sulfur can be separated by using a magnet to attract the iron. Iron is a magnetic material but sulfur is not. If a mixture of iron filings and sulfur is heated the iron reacts with the sulfur and the compound iron sulfide is formed. The compound iron sulfide is not a magnetic material and cannot be separated by using a magnet. Separation Techniques - Physical Methods. 1) Filtration 2) Crystallisation 3) Distillation 4) Chromatography Mixtures - Separation Techniques - Filtration. A solid which has not dissolved in a liquid can be separated by filtration. A filter paper is placed inside a glass funnel and a container put beneath. Filtration The solid remaining in the filter paper is called the residue. The residue can be dried by spreading it out on the filter paper and allowing the liquid to evaporate. The liquid which has passed through the filter paper is called the filtrate. Mixtures - Separation Techniques - Crystallisation. A solid which has dissolved in a liquid (called a solution) can be separated by crystallisation. The dissolved substance is called the solute. The liquid used for dissolving is called the solvent. The solution is warmed in an open container, allowing the solvent to evaporate, leaving a saturated solution. A solution which has as much solid dissolved in it as it can possibly contain, is called a saturated solution. As the saturated solution is allowed to cool, the solid will come out of the solution and crystals will start to grow. The crystals can then be collected and allowed to dry. Mixtures - Separation Techniques - Fractional Distillation. A liquid can be separated from a mixture of liquids in a solution by fractional distillation. 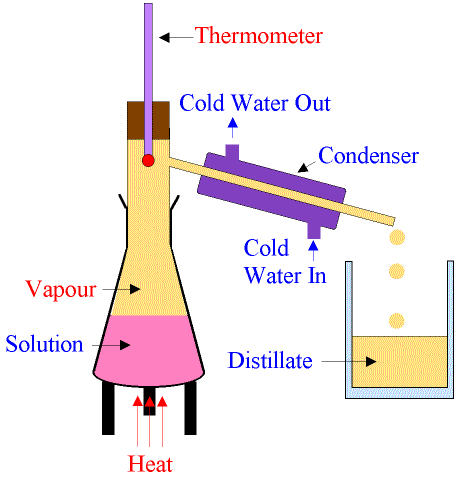 The solution is heated until it boils. The liquid with the lowest boiling point boils first and becomes a vapour (gas). The vapour is cooled in the condenser until the temperature falls below the boiling point when it condenses back into a liquid which is collected in a container. The collected liquid is called the distillate. It has been distilled. The condenser has cold water running through a jacket around the outside to keep the temperature below the boiling point of the vapour. After the liquid with the lowest boiling point has been collected, the temperature of the remaining mixture will rise to a new temperature when the liquid with the next lowest boiling point will boil and can be collected. The process can be continued to separate all the liquids in the mixture (see also the fractional distillation of crude oil). Mixtures - Separation Techniques - Chromatography. 
This technique is called paper chromatography. The mixture (in this case two green ink spots) is put on a filter paper which is placed in a suitable solvent. As the solvent rises up the filter paper the individual dyes within the green ink spots are separated and different dyes travel different distances up the paper. The solvent travels furthest up the filter paper leaving a line called the solvent front. In the above example the green ink spots each have the same blue dye (because they have travelled the same distance) but different yellow dyes (because they have travelled a different distance). Solid, Liquid and Gas. Any substance may exist as solid, liquid or gas. If a solid is heated, it will melt to become a liquid. The temperature at which it melts is called its melting point. If the liquid is then cooled, it will freeze to become a solid again. The temperature at which it freezes is called its freezing point. The melting point and the freezing point is the same for the same substance. Similarly, if a liquid is heated it will boil to become a gas. The temperature at which it boils is called its boiling point. If the gas is then cooled, it will condense to become a liquid again. A gas will condense at its boiling point. Sometimes a heated solid will turn into a gas without becoming a liquid. This is called sublimation. Examples of solids which sublime are iodine and carbon dioxide. The figure below shows interconverting from one state to another by heating or cooling. Red arrows involve heating, blue arrows involve cooling. The state of a substance (whether it is solid, liquid or gas) depends on its temperature, the RFM of the particles and the forces of attraction between the particles. Solid, Liquid and Gas - Particle Motion. Solid. In a solid, the particles can vibrate but they cannot move from one place to another. As the solid is heated, the particles vibrate more and more until the force of attraction between them is overcome. The temperature at which this happens is called the melting point. Above this temperature, the solid has become a liquid. Liquid. In a liquid the force of attraction between the particles is weaker than it is in the solid. It is still strong enough that the particles are held close to each other but they are now free to move. As the liquid is heated, the particles move faster and faster until they overcome the force of attraction between them. The temperature at which this happens is called the boiling point. Above this temperature, the liquid has become a gas. Gas. A gas takes up a lot more space (occupies a greater volume) than the boiling liquid it came from. This is called expansion. In a gas, the particles move fast in random directions. There is no force of attraction between the particles. Solid, Liquid and Gas - Structure and Properties. Heatingarrow A solid has a regular arrangement of particles (atoms, ions or molecules). The particles are close together and cannot move around. The shape of a solid is fixed but the volume will increase as the temperature increases. This happens because the particles vibrate more and move a little further apart causing a decrease in density. A solid cannot be compressed because the particles are already close together. It is said to have long range order which means that the arrangement keeps repeating itself for a huge number of particles. A liquid has an arrangement of particles which are close together but they are free to move because the force of attraction between the particles is weaker than it is in a solid. A liquid will flow to take the shape of its container. Its volume changes with temperature (see solid above). It cannot be compressed because the particles are already close together. It is said to have short range order which means that the arrangement only repeats itself for a small number of particles. A gas has no order, its particles are arranged at random. A gas will fill the whole volume of its container. The speed of the particles in a gas increases as the temperature increases. This causes the pressure of a gas to increase. Its particles are so far apart that there is no force of attraction between them. The particles are unaware of each others existence unless they collide. A gas is easily compressed. The graph below shows how the temperature changes with time as a substance is heated at a constant rate. There are obvious flat sections of the graph at the melting and boiling point.  At the melting point, although the substance is still being heated, there is a time when the temperature does not change. During this time, all the extra heat which is being added goes to overcome the force of attraction (the bonds) between the particles of the solid as it turns into a liquid. The temperature of a solid can never be raised above its melting point at atmospheric pressure. When all the solid has melted, then the temperature of the liquid will start to rise Solid, Liquid and Gas - Melting and Boiling At the boiling point, the temperature of a liquid does not change until all the liquid has boiled and become gas. During this time, the extra heat energy goes into overcoming the force of attraction between the particles of the liquid. The temperature of a liquid can not be raised above its boiling point at atmospheric pressure. The same graph (as the previous page) occurs on cooling a gas to a liquid, or a liquid to a solid. The process of melting or boiling requires extra energy - it is endothermic. The process of freezing or condensing gives out energy - it is exothermic. Brownian Motion. All matter is made of tiny particles. The particles are atoms, ions or molecules. In a liquid or gas, the particles move at random. The random motion of particles (called Brownian Motion) can be seen by looking at smoke particles in air through a microscope.  The smoke particle (shown as a blue ball) has a jerky motion. As (invisible) air molecules collide with the smoke particle, they push it about in different directions at random. Diffusion. When two gases or liquids mix together, the fast randomly moving particles collide, and this slows down the rate of mixing. Diffusion is the random movement of one liquid or gas through another from a region of high concentration to a region of low concentration. Potassium Manganate (VII) is dark purple. When the solid is dissolved in water, the purple particles slowly diffuse out into the clear liquid.  Eventually, the random motion of all the particles results in the purple colour being equally dispersed throughout the water. Note that the particles are moving fast but the many collisions with each other slow their progress, and so diffusion appears to be slow. Diffusion of Bromine through Air. Bromine is a red-brown liquid that boils at 58 °C. Bromine gas is also red-brown and can be seen inside a gas jar. If bromine gas is allowed to enter a gas jar containing a vacuum, the red-brown colour instantly fills the jar because there are no air molecules to collide with. This shows that the bromine molecules are moving fast. When bromine is added to a gas jar filled with air, the red-brown gas is seen to slowly diffuse through the air. The many collisions between the bromine molecules and the air molecules slow down the rate at which the red-brown bromine gas fills the jar. Diffusion of Hydrogen Chloride and Ammonia. Cotton wool soaked in concentrated ammonia solution, NH3(aq) and concentrated hydrogen chloride solution (hydrochloric acid) HCl(aq) are placed at each end of a sealed tube. The cotton wool with ammonia solution gives off ammonia molecules (NH3). The cotton wool with hydrochloric acid gives off hydrogen chloride molecules (HCl).  HCl and NH3 molecules diffuse through the air towards each other. When they meet, they react to form a white powder called ammonium chloride, NH4Cl. hydrogen chloride + ammonia ammonium chloride. HCl(g) + NH3(g) NH4Cl(s) The sign shows that the reaction is reversible. The ring of white powder is closer to the HCl than the NH3. This is because the NH3 molecules are lighter (smaller) and have diffused more quickly through the air in the tube (you can work out which molecule is lighter by looking at the RFM). Note that lighter (smaller) particles move more quickly than heavier (larger) ones. Chemical and Physical Properties. The phrase "chemical properties" describes the way that an element or compound reacts chemically with other substances. The chemical properties are determined by the number of electrons in the outer shell of the atom. This is the same as an elements group number in the periodic table. Physical Properties. The physical properties of an element or compound (any material) are 1) Melting and boiling point. 2) Density. 3) Conduction of Electricity. 4) The size of an atom, ion or molecule, and its rate of diffusion. 5) Strength, stiffness, hardness and elasticity. Strength is a measure of the amount of force needed to pull a material apart (called tensile strength) or push it until it is squashed (called compressive strength). Stiffness is a measure of the amount of force needed to change the shape of an object by bending it. When the force is removed the object returns to its original shape. This is an example of elastic deformation. Hardness is a measure of the amount of force needed to change the shape of an object by bending or scratching it. When the force is removed the object does not return to its original shape. This is an example of permanent deformation. The word hardness is also used to mean something completely different when describing water. Quantitative and Qualitative. Quantitative means describing a reaction in terms of the amounts (quantities) of substances. Qualitative means describing a reaction in terms of what it does. For example, when magnesium burns in air, a qualitative description would be "Magnesium burns in air with a brilliant white flame to form magnesium oxide" a quantitative description would be "10g of magnesium burns in air to form 16·67g of magnesium oxide" (see moles).Hazard Symbols. The following symbols are sometimes found on containers. These symbols mean that the substance is dangerous. The explanation for the symbol is given below.  Oxidising - The substance provides oxygen. Other materials will burn more fiercely in its presence. Highly Flammable - The substance will catch fire easily. Toxic - The substance is poisonous and can kill. Possible routes into the body are breathing, swallowing and absorption through the skin. Harmful - Similar to toxic but less dangerous. Corrosive - The substance will attack and destroy living tissue, including the skin and eyes. Irritant - The substance is not corrosive but may cause reddening, irritation or blistering of the skin. Matter:Classification of matterIs distilled water a solution? Is fire matter? Properties of matter What are extensive and intensive properties? What is the difference between chemical and physical change? States of matterWhat are some examples of plasma?
Atoms, elements, and ions:
What's the difference between Na+ and Na?
|
Measurement:
Why isn't 0°F the lowest possible temperature for a salt/ice/water mixture? Density What is a "degree Baumé"? How can density be found by water displacement? How can I estimate the density of a liquid mixture (e. g. liquid air?) Does ice weigh more or less than water? How much sand should Jones have used in Raiders of the Lost Ark? Can I get a solution density from the solute mass and solvent volume? What is specific gravity? How can the mass of air in a room be computed from the room's dimensions? How do I find water's density at different temperatures? Scientific notationHow is a number converted to scientific notation?Significant figures Why should the rules for propagating significant digits not be applied to averages? Why does 1101 cm - 1091 cm = 10 cm with 2 significant figures? Are there simpler rules for counting significant digits? Unit conversionAt what temperature are Fahrenheit and Celsius temperatures equal?When is it wrong to build conversion factors from given information? How do I find grams of solute in a volume of solution, given parts per million? UnitsIs the SI the same as the metric system?Which is correct: decameter (dc) or dekameter (dk)? What are reciprocal units? Why is the difference between Celsius and Kelvin temperatures 273 units?
Chemical change:
Why is H+ sometimes written as H3O+ in equations?Can you classify acids as strong or weak from their formulas alone? Chemical equationsHow can I tell if an equation is balanced correctly?What are some examples of reactions that involve catalysts? Combustion reactionsHow do I balance the equation for combustion of Si2H6?What is the balanced equation for combustion of octane? Is oxygen flammable? Double displacement reactionsHow do I complete and balance an equation for reaction of NaOH with HCl?How should the reaction between vinegar and baking soda be classified? How do I write a net ionic equation for an aqueous double displacement reaction? How do I predict products given only the formulas of the reactants? ElectrolytesAre nonelectrolytes always nonpolar?Redox reactionsHow can I recognize redox reactions?Solubility rulesWhat are everyday applications for the chloride solubility rule?
The mole concept:
How many N atoms are in 2.05× 1022 N2O molecules?How many atoms or moles of an element are in one mole of compound? GravimetryWhy should AgCl be washed with dilute HNO3 in a gravimetric analysis?Molecular weightHow can I find molecular weights for specific substances?Reaction stoichiometryHow can I use the amount of fuel to predict amount of product in a combustion reaction?How can amount of product (KNO3) be predicted from amounts for two reactants (KCl, HNO3) How do I calculate the "expected yield" for a reaction? How do I compute mass of product (O2) from mass of reactant (KClO3)? How much alum can be prepared from a given mass of aluminum? How do I compute yield for multistep synthesis (Cr(acac)3)? Solution stoichiometryHow many grams of solute are in a certain volume of a solution of known molarity?What is the molarity of salt in seawater? The moleHow can I construct a metaphor that shows how huge Avogadro's number is?How can I convert moles to milliliters? Moles confuse me- why are they used? TitrationsHow can I adjust the pH of an HNO3 solution by adding KOH?How can Ba(OH)2 concentrations be determined using H2SO4, HCl, and Na2SO4 solutions? How can I find the molecular weight of an organic acid? What is a "standard solution"? How do I find percentage iron in an ore, given KMnO4 titration data? Gases:Avogadro's Law and gas densitiesWhat volume of gas is produced by vaporizing a given volume of dry ice? How do I estimate gas densities at STP? How do I predict whether a gas is heavier or lighter than air? How many molecules are present in a given volume of gas at STP? Why is wet air less dense than dry air at the same temperature? Empirical gas laws What are some examples of the gas laws in action in everyday life? How does the volume of gas in a pop bottle change on opening the bottle? What volume of gas is needed to prepare a 20% NH3 solution? Mixtures of gases What is the final pressure when two gases at different pressure are mixed? Molecular weights of gases How can I find the molecular formula of a gas from experimental data? Reaction stoichiometry How much gas is produced when baking soda react with excess vinegar? What volume of gaseous reactant produces a given amount of product? Real gases Under what conditions do real gases behave ideally?
Energy and chemical change:
How do I calculate calorimeter heat capacities from experimental data?How do I calculate the amount of ice needed to cool water to a certain temperature? How can I calculate the energy required to cause a temperature rise? How can the heat capacity of air be estimated? How does the ice in coolers stay cool? EnergyHow can energy be changed from one form to another?Why do different types of change involve different amounts of energy? EnthalpyWhy can we measure enthalpy changes, but not absolute enthalpies?How can enthalpy changes for a reaction be estimated from bond energies? What are some examples of exothermic and endothermic processes? What is Hess's Law? ThermochemistryWhy does mixing a strong acid with water release so much heat?Why does dehydration of sugar by acid produce so much heat? How can I calculate the heat released from the dehydration of sugar by sulfuric acid? Where can I find thermochemical data for specific compounds? What are the properties of a good rocket fuel? Is heat absorbed or released when vinegar reacts with baking soda? Why are metal displacement reactions exothermic?
The quantum theory:
How does electron confinement in an ion or molecule affect its color?
Radiation and matterWhat makes a compound optically active?How is photoelectron energy related to excitation wavelength? The uncertainty principleIf electrons can't be confined to the nucleus, why does K-electron capture occur?Why don't electrons get stuck in the nucleus?
Electrons in atoms:
Which has a line spectrum most like that of hydrogen: Li, Li+, or Li2+?
|
