Fractional Distillation
What is fractional distillation?
Fractional Distillation is a separation technique that is used
for liquids that dissolve in each other.
Separation process in which the volatile components (having different boiling points) of a mixture (such as crude oil) are split from one another by heating the mixture in a column and collecting and condensing the vapors drawn from different levels of the column.
Fractional distillation is used to separate
the components of crude oil
and to separate nitrogen and oxygen from liquid air.
Immiscible liquids are separated using a separating funnel.
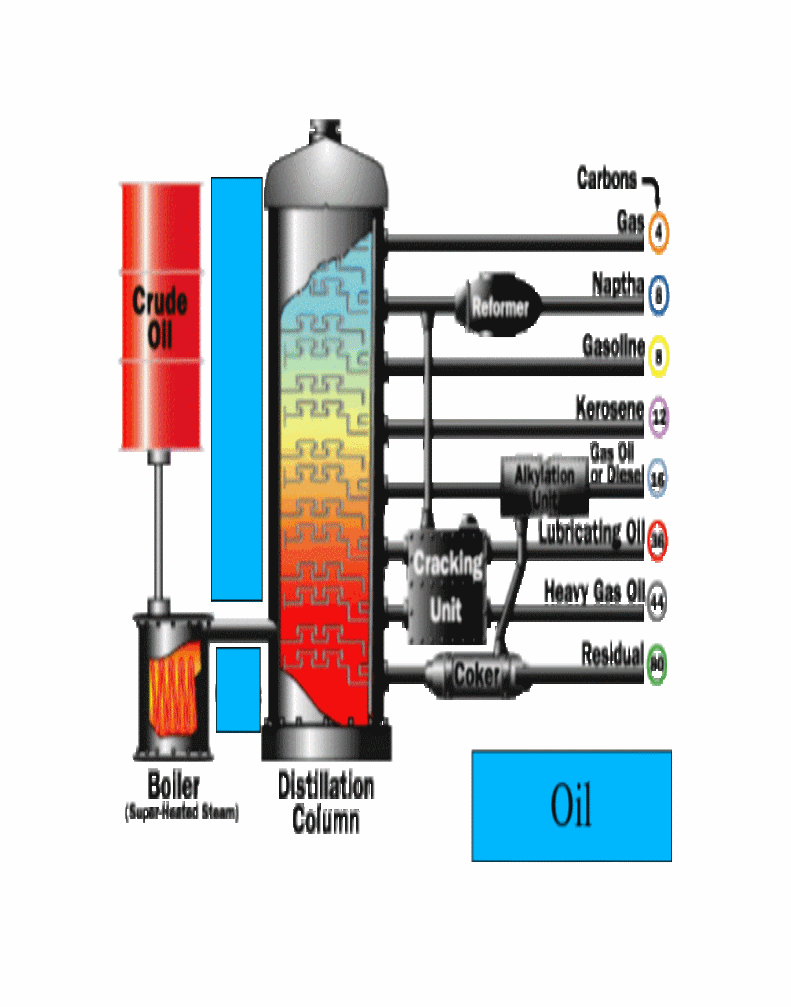
The various components of crude oil have different sizes, weights and boiling temperatures; so, the first step is to separate these components. Because they have different boiling temperatures, they can be separated easily by a process called fractional distillation. The steps of fractional distillation are as follows:
1.You heat the mixture of two or more substances (liquids) with different boiling points to a high temperature. Heating is usually done with high pressure steam to temperatures of about 1112 degrees Fahrenheit / 600 degrees Celsius.
2.The mixture boils, forming vapor (gases); most substances go into the vapor phase.
v
3.The vapor enters the bottom of a long column (fractional distillation column) that is filled with trays or plates. The trays have many holes or bubble caps (like a loosened cap on a soda bottle) in them to allow the vapor to pass through. They increase the contact time between the vapor and the liquids in the column and help to collect liquids that form at various heights in the column. There is a temperature difference across the column (hot at the bottom, cool at the top).
4.The vapor rises in the column.
5.As the vapor rises through the trays in the column, it cools.
6.When a substance in the vapor reaches a height where the temperature of the column is equal to that substance's boiling point, it will condense to form a liquid. (The substance with the lowest boiling point will condense at the highest point in the column; substances with higher boiling points will condense lower in the column.).
7.The trays collect the various liquid fractions.
8.The collected liquid fractions may pass to condensers, which cool them further, and then go to storage tanks, or they may go to other areas for further chemical processing
Fractional distillation is useful for separating a mixture of substances with narrow differences in boiling points, and is the most important step in the refining process.
(Do you have better answer?)
(Do you have better refinery?)
(Does anyone else have a better answer?)
(Does anyone else have a better refinery?)
How does this transformation take place?
Essentially, refining breaks crude oil down into its various components, which then are selectively reconfigured into new products. All refineries perform three basic steps: separation, conversion, and treatment.
Separation:
Heavy petroleum fractions are on the bottom, light fractions are on the top. This allows the separation of the various petrochemicals. Modern separation involves piping oil through hot furnaces. The resulting liquids and vapors are discharged into distillation towers.
Inside the towers, the liquids and vapors separate into components or fractions according to weight and boiling point. The lightest fractions, including gasoline and liquid petroleum gas (LPG), vaporize and rise to the top of the tower, where they condense back to liquids. Medium weight liquids, including kerosene and diesel oil distillates, stay in the middle. (Heavier liquids, called gas oils, separate lower down, while the heaviest fractions with the highest boiling points settle at the bottom.)
Conversion:
The finishing touches occur during the final treatment. To make gasoline, Cracking and rearranging molecules adds value to the products. This is where refining's fanciest footwork takes place--where fractions from the distillation towers are transformed into streams (intermediate components) that eventually become finished products. The most widely used conversion method is called cracking because it uses heat and pressure to "crack" heavy hydrocarbon molecules into lighter ones. A cracking unit consists of one or more tall, thick-walled, bullet-shaped reactors and a network of furnaces, heat exchangers and other vessels.
Cracking and coking are not the only forms of conversion. Other refinery processes, instead of splitting molecules, rearrange them to add value. Alkylation's, for example, makes gasoline components by combining some of the gaseous byproducts of cracking. The process, which essentially is cracking in reverse, takes place in a series of large, horizontal vessels and tall, skinny towers that loom above other refinery structures. Reforming uses heat, moderate pressure and catalysts to turn naphtha, a light, relatively low-value fraction, into high-octane gasoline components.
Treatment:
The finishing touches occur during the final treatment. To make gasoline, refinery technicians carefully combine a variety of streams from the processing units. Among the variables that determine the blend are octane level, vapor pressure ratings and special considerations, such as whether the gasoline will be used at high altitudes.
Storage:
These liquids are stored in large tanks on a tank farm. Pipelines carry the final products from the tank farm near the refinery to other tanks all across.
All of these activities are required to make the gasoline that powers our cars, the diesel fuel that brings our food to market, and the jet fuel that flies our planes. These provide us with the energy we need to get from place to place quickly and comfortably.
Question: What are the products and uses of petroleum?
The most common products from petroleum are energy products: gasoline, heating oil, and diesel fuel. Other petroleum products are: ink, crayons, bubble gum, dishwashing liquids, deodorant, eyeglasses, records, tires, ammonia, and heart valves.
A barrel of oil yields these refined products (percent of barrel):
47% gasoline for use in automobiles
23% heating oil and diesel fuel
18% other products, which includes petrochemical feedstock's products derived from petroleum principally for the manufacturing of chemicals, synthetic rubber and plastics
10% jet fuel
4% propane
3% asphalt
Question: Can I tell which country the gasoline at my local station comes from?
The Energy Information Administration does not collect data on the source of gasoline sold at retail outlets. Several factors make it difficult to say where gasoline at a local station originated:
At a local station, a company may sell gasoline that was not produced by its own refineries.
Gasoline from different refineries, owned by different companies, is often combined for shipment by pipeline. Many companies may purchase gasoline at the same bulk terminal.
Where Does My Gasoline Come From?
America consumes over 20 million barrels (840 million gallons) of petroleum products each day, almost half of it in the form of gasoline used in over 200 million motor vehicles with combined travel over 7 billion miles per day. Gasoline is made from crude oil. Refineries break down these hydrocarbons into different products. These refined products include gasoline, diesel fuel, heating oil, jet fuel, liquefied petroleum gases, residual fuel oil, and many other products .
The characteristics of the gasoline produced depend on the type of crude oil that is used and the setup of the refinery at which it is produced. Gasoline characteristics are also impacted by other ingredients that may be blended into it, such as ethanol. The performance of the gasoline must meet industry standards and environmental regulations that may depend on location.
South Asia, Africa, Europe, Middle East.
Precise details, coming soon.
How many oil companies are there in North America?
Shell Oil Co.,
BP America Inc.,
Chevron Corp.
ConocoPhillips.
------------------
(Do you have better answer)
Distribution
|
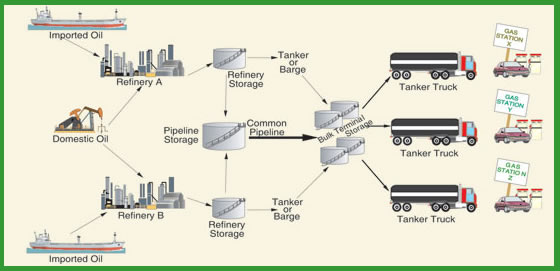
From Refinery to Consumer
After crude oil is refined into gasoline and other petroleum products, the products must be distributed to consumers. The majority of gasoline is shipped first by pipeline to storage terminals near consuming areas, and then loaded into trucks for delivery to individual gas stations. Gasoline and other products are sent through shared pipelines in "batches." Since these batches are not physically separated in the pipeline, some mixing or "commingling" of products occurs. This is why the quality of the gasoline and other products must be tested as they enter and leave the pipeline to make sure they meet appropriate specifications. Whenever the product fails to meet local, state, or federal product specifications, it must be removed and trucked back to a refinery for further processing.
After shipment through the pipeline, gasoline is typically held in bulk storage terminals that often service many companies. At these terminals the gasoline is loaded into tanker trucks destined for various retail gas stations. The tanks in these trucks, which can typically hold up to 10,000 gallons, usually have several compartments, enabling them to transport different grades of gasoline or petroleum products. The truck tank is where the special additive packages of gasoline retailers get blended into the gasoline to differentiate one brand from another. In some areas, ethanol may be splash blended in the tanker to meet environmental requirements. When the tanker truck reaches a gas station, the truck operator unloads each grade of gasoline into the appropriate underground tanks at the station.
Grades and Formulations
Service stations usually sell several grades of gasoline: premium, mid-grade, and regular. These grades have different "octane ratings" which reflect the gasoline's anti-knock properties. The owner's manual for your car tells you what grade of gasoline your car needs. Most cars can run on regular gasoline, which is the cheapest.
|
|



