Aluminum
|
How many elements are there?
What should you know about a chemical element? What is the name of the chemical element and its derivatives that you are manufacturing? What do you understand by derivatives of an element? Is there a difference between a compound and an alloy? What is the difference between a compound and an alloy? What do you know about the chemical element aluminum? What should you know about this element? What are the properties of this element? How is the pure aluminum element manufactured? What common shapes of the pure aluminum element are manufactured? How is this element extracted? How is this element recycled? What are the compounds of this element? What are the alloys of this element? What is the history of this element? What are the health concerns of this element? What effects does this element have on plants? What are the uses of this element? What products are manufactured from this chemical element or its derivatives? What are the alloys relevant to this element? What are names and details of the chemical elements in alphabetical order? What should you know about a chemical element? Can you elaborate on a chemical element in 222 questions and answers? What is the name of this chemical element? What is the symbol of this chemical element? What is the atomic number of this chemical element? The atomic number and serial number are usually the same. What is the atomic weight of this chemical element? What is the standard state of this chemical element? Is it solid, liquid, or gas? What is the standard CAS Registry ID of this chemical element? What is the group of this chemical element? What is the group name of this chemical element? What is the period of this chemical element? What is the block of this chemical element? What is the color of this chemical element? Is this chemical element metallic of non-metallic? What are uses of this chemical element naturally? What does this chemical element react with? How do you calculate the atomic number? What products are made from this chemical element? |
|
Elements
How many elements are there? http://www.qureshiuniversity.com/elements.html What should you know about a chemical element? You should be able to elaborate on chemical elements under at least 100 different headings. Atomic number Atomic mass and atomic weight Allotropes Abundance Alloys Appearance Atomic Radius (pm) Atomic Volume (cc/mol) Abundance of elements (Earth's crust) Abundance of elements (oceans) Abundance of elements (meteorites) Abundance of elements (stream) Abundance of elements (sun) Abundance of elements (Universe) Abundances in humans Accurate mass of the isotopes Biological role Block in periodic table Boiling point Bond enthalpy (diatomics) Bulk modulus Covalent Radius Chloride(s) Crystal structures Chemical symbols Compounds Covalent radius 2008 values Covalent radius Critical temperature Crystal structure |
| CAS Registry Number |
|
Density (g/cc) Description Discovery Debye Temperature (K) Extraction Effect on plants Effective nuclear charge Electrical resistivity Electron affinity Electron binding energies Electronegativities Electronic configuration Element bond length Enthalpy of atomization Enthalpy of fusion Enthalpy of vaporization Examples of compounds Element Classification: Transition Metal Evaporation Heat (kJ/mol) Electrical Resistivity (20°C) Fusion Heat (kJ/mol) First Ionizing Energy (kJ/mol) Group numbers Hardness _ Brinell Hardness _ Vickers Hardness _ Mohs Hydride(s) History of the element Health concerns Isotopes Ionic atom_sizes (Shannon) Ionic radius (Pauling) Ionic radius (Pauling) of monocation ionisation energies Isolation Isotope abundances Isotope nuclear spins Isotope nominal mass Isotope nuclear magnetic moment Lattice Structure Lattice Constant (Ć) Lattice energies Linear expansion coefficient Melting and boiling points Meaning of name Melting point Mineralogical hardness Molar volume Magnetic Ordering Names and symbols NMR frequency NMR isotopes NMR magnetogyric ratio NMR quadrupole moment NMR receptivity NMR relative sensitivity Nomenclature and symbols Occurrence and origin on Earth Oxide(s) Oxidation States Properties Recycling Production and refinement Poisson Ratio Pauling Negativity Number Poisson's ratio Properties of some compounds Radius metallic (12) Radioactive isotopes Reactions of elements Reduction potential M(aq) Reflectivity Refractive index Registry number Rigidity modulus Reaction with Air Reaction with 6M HCl Reaction with 15M HNO3 Standard atomic weight Standard state Solid, liquid, or gas Superconductivity temperature Symbol Sources Speed of Sound Specific Heat Shear Modulus Term symbol Thermal conductivity Thermal Expansion (25°C) Thermodynamic properties Uses Valence orbital R(max) Van der Waals radius Velocity of sound X_ray crystal structure Young's modulus What is the name of the chemical element and its derivatives that you are manufacturing? Aluminum What do you understand by derivatives of an element? Derivatives can be compounds, alloys, other combinations. Is there a difference between a compound and an alloy? Yes, there is. What is the difference between a compound and an alloy? A compound is a chemical substance that consists of two or more elements that together form a molecule. An alloy mixture contains two or more metallic elements or metallic and non-metallic elements that are usually fused together or dissolving into each other when molten. What should be the focus during studies of the properties and uses of elements? www.qureshiuniversity.com/elementsgeneral.html What is aluminum? Chemical & Physical Properties What are properties of the element? Extraction of Aluminium How is the element extracted? 16 What is Aluminium Ore called? 17 What is the Chemical Formula for Aluminium Oxide? 18 Why is Cryolite added to Aluminium Ore? 19 Which Gases are given off at the Anode? 20 Why does the Anode need to be Replaced? 21 Write the Balanced Equation for the Overall Reaction. 22 Why is Aluminium More Expensive than Iron? 23 Why is Aluminium Resistant to Corrosion? 24 What is Anodising? 25 What Substance is used to Remove the Oxide Layer before Anodising? 26 Which Acid is used as the Electrolyte for Anodising? 27 Which Gas is given off at the Anode during Anodising? 28 Which Gas is given off at the Cathode during Anodising? 29 Does Aluminium have a High Density? 30 Is Aluminium a Good Conductor of Heat? 31 Give two uses of Aluminium. http://www.gcsescience.com/ex39.htm Castings Forgings Uses What are the uses of the element? What is aluminum used for? |
|
Properties Production and refinement Recycling Compounds History Health concerns Effect on plants General use |
| Aluminum |
| Manufacture |
| Aluminum Questions |
|
What's aluminum like? What's aluminum used for? How is aluminum made? |
| What is aluminum? |
|
Aluminium or Aluminium Facts Chemical & Physical Properties What are properties of the element? www.qureshiuniversity.com/aluminiumproperties.html |
| Extracting aluminium from bauxite |
| What is aluminum used for? |
| Aluminium foil |
| Aluminum Foil |
| Aluminum Food Containers |
|
Aluminum Foil The Manufacturing Process |
| http://www.youtube.com/watch?v=f4OTj9yNOak |
| Aluminium in The Building and Construction |
 Personal Care
Personal Care Beverage Food Household Pharmaceuticals Automotive |
| Closures Aluminium (General use) |
| Aluminium (General use) |
| Aluminium Properties |
| How is aluminium recycled? |
| Aluminium Engine |
|
Overview Transportation
Aircraft Marine Rail Space
Foil Rigid Containers Other Consumer Products
Cable/Transmission Commercial Domes
-Alloys -Castings -Extrusions -Foil -Forgings -Impacts -Ingot, Billet -Molten Metal, T-Bar, Sow -Powder and Paste -Sheet, Plate -Wire, Rod & Bar Environment |
| Aluminum Wire, Rod, Bar & Tube |
|
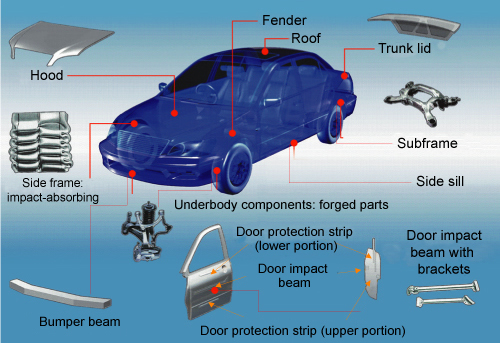
Brief description: pure aluminium is a silvery-white metal with many desirable characteristics. It is light, nontoxic (as the metal), nonmagnetic and nonsparking. It is somewhat decorative. It is easily formed, machined, and cast. Pure aluminium is soft and lacks strength, but alloys with small amounts of copper, magnesium, silicon, manganese, and other elements have very useful properties. Aluminium is an abundant element in the earth's crust, but it is not found free in nature. The Bayer process is used to refine aluminium from bauxite, an aluminium ore.
The first step in extracting aluminium is to remove it from the Earth in mining. This is relatively simple given the abundance of the material. However aluminium is never found isolated in the Earth (due to its reactivity) but instead it is found bound to other elements in compounds. This means that aluminium alone in can never be dug up, but compounds of it, often containing oxygen and silicon, are. Bayer process on industrial scale The bauxite then has to be purified using the Bayer process, whose development changed the course of aluminium's history. The process occurs in two main steps. Firstly the aluminium ore is mixed with the sodium hydroxide in which the oxides of aluminium and silicon will dissolve, but other impurities will not. These impurities can then be removed by filtration. Carbon dioxide gas is then bubbled through the remaining solution, which forms weak carbonic acid neutralising the solution and causing the aluminium oxide to precipitate, but leaving the silicon impurities in solution. After filtration, and boiling to remove water, purified aluminium oxide can be obtained. The Hall Heroult Process Once purified aluminium oxide has been manufactured aluminium can be removed from it by the Hall-Heroult Method. In this the aluminium oxide is mixed with cryolite (made of sodium fluoride and aluminium fluoride) and then heated to about 980 °C to melt the solids. This is much lower than the temperature required to melt pure aluminium oxide so much energy is saved. The molten mixture is then electrolysed with a very large current and the aluminium ions are reduced to form aluminium metal (at the cathode) and oxygen gas is formed at the anode, where it reacts with the carbon the anode is made from to give carbon dioxide gas. As the process is so long and requires so much energy (in electricity) the aluminium metal obtained is quite expensive, but still it is competitively priced in relation to other metals unlike earlier in its history. One of aluminium's weaknesses is it's lack of strength is its pure form. To get around this and preserve aluminium's low density and lightweight other elements are added to the metal to "pin" dislocations reducing ductility but increasing strength. By this method some aluminium alloys can be as strong as steel. Adding different elements achieves slightly different effect but almost all alloys are stronger than the original aluminium metal. Copper, an important part of some aluminium alloys Adding copper to aluminium increases aluminium's strength, and hardness and also makes it heat treatable. Under a classification system that is currently in place all aluminium alloys are give a four digit number. Those with copper come in the form 2XXX. Alternatively adding magnesium causes increased tensile strength, resistance to marine corrosion and ease at which welding can occur. The code for these alloys begins with a 5. Manganese also forms important aluminium alloys. There are three further common elements which can feature in aluminium alloys. Manganese is often added to give increased strength and resistance to corrosion. The addition of silicon lowers the melting point and improves castability, and alloys with zinc have increased strength and hardness. What makes these alloys really special is they retain the lightweight property of aluminium whilst gaining the extra properties that aluminium itself lacks. A dislocation is pinned by a different element in an alloy, increasing strength But how do the addition of these materials strengthen aluminium? It is all to do with aluminium's structure which has dislocations which make it ductile, and malleable. Even though these properties are sometimes very useful often strength is more important and alloys use other elements (usually of different sizes) to stop the dislocations from moving. This is done by disrupting the regular crystal structure and therefore making it harder for atoms to slip past each other. Most of the uses that aluminium has are in fact as part of an alloy rather than as a pure metal. Pure aluminium simply isn't strong enough for usage in aeroplanes, cars, trains and buildings. Aluminium conducts electricity well (and is lightweight) making itself ideal for uses in long distance electrical transmission. This is a property that it shares with other metals, and it conducts electricity for the same reasons that they do -- free electrons. The same free electrons that give reflectivity. Positive ions in a sea of free electrons The electrons in metal atoms become delocalised in a metal crystal forming a regular lattice of positive ions surrounded by electrons. These electrons are free too move. Therefore when a potential difference is applied to a metal the electrons are attracted to the positive end and as they are free they can move in that direction. At the same time more electrons are being added to the metal at the negative end. So in this way electricity is conducted. Another model of conductivity is called the electron well. This is based on the energy required to remove an electron from an atom. The idea is that in metals there are more electrons that have enough energy to move across the whole metal (ie. are in the "conduction band") than in insulators. Aluminium has a particularly large amount of electrons in the conduction band as it is such a good conductor (it has a very low resistivity: 2.65 x 10-8 Ohm metres). With heat conductivity it works in a similar way. In non-metallic elements heat is transferred by vibrations of atoms causing the next atom to vibrate, and then the next, and so on. In this was the heat moves by conduction. In metals this process also occurs, but the delocalised electrons can also move around to pass on the heat energy. This greatly speeds the process up. Therefore as aluminium is a good electrical conductor it is also a good conductor of heat. This makes aluminium ideal in kitchen pans as it heats that food quickly, and evenly. As is made clear in the uses section, aluminium's corrosion resistance plays a vital role in many of its uses, from packaging, to jumbo jets, and to building and construction work. When exposed to air aluminium forms a 1 nm thick layer of oxide which serves as a brilliant corrosion protector for the metal in most environments. The effect of oxygen and time on iron, aluminium is not affected like this. The barrier oxide film is bonded very strongly with the surface, and if it does become damaged it reforms almost immediately in air, maintaining this very high level of protection. This oxide layer changes the properties of the material hugely. Although aluminium is a very reactive metal, in most case this reactivity cannot be seen due to this natural film which prevents the aluminium reacting with other substances. Unlike in iron where the oxidation of the metal creates substance (rust) damaging to the metal itself, in aluminium the oxide makes the material even more versatile and useful. Aluminium would not be used to the extent that it is in outdoor furniture, gutterings, cars, planes, and on buildings if it couldn't protect itself from the effects of weathering and corrosion. With this layer, it can. Hardness: Hardness is the ability to not be easily scratched, in is measured in Pascals or force per unit area. Aluminium's hardness is relatively low due to its low density (see below). This means that it is easier to scratch than other metals like steel are. Aluminium Density: Low 2700 kg / cubic metre This gives aluminium its unique lightweight property and extends the uses of aluminium vastly. Really this is the key property that sets aluminium apart from so many other metallic elements. Transport vessels made of aluminium can carry more cargo with the same amount of fuel than vessels made of other materials as the aluminium itself it so much lighter. Why does it have such a low density? Because of the number of protons and neutrons (which make up almost all of an atoms weight) in the nucleus. Aluminium has a mass of just 27, which is very low compared to most other metals. Melting Point: 660.32 °C Boiling Point: 2519 °C These relatively low thermal points help aluminium to be reshaped, and welding quite easily. For a metal a melting point of 660.32 °C is very low. Aluminium in pylons Electrical Resistivity: Low 2.65 x 10-8 Ohm metres Aluminium has a very low electrical resistivity, and therefore a high electrical conductivity. This is measured in Ohms meters, as resistivity is equal to the resistance of a certain sized piece of the material multiplied by the area and divided by the length. This gives a standard measure for a material that can be used easily in comparison. In relation to other metals aluminium has quite a low resistivity, it is not the best conductor, but it is by no means the worst. Aluminium is also a very good thermal conductor as is discussed in conductivity. Reflectivity: High 71% unpolished and when polished: 97% Aluminium is extremely reflective, when polished (some sources say) it is the most reflective material, for this reason it is now frequently used in lights. It is also very heat reflective and because of this people use it in their car windscreens to reflect the heat, and to keep the car cool on sunny days. The aluminium industry relies on the Bayer process to produce alumina from bauxite. It remains the most economic means of obtaining alumina, which in turn is used for the production of aluminium metal - some two tonnes of alumina are required to produce on tonne of aluminium. The primary aluminium industry is dependent on a regular supply of alumina for four functions: basic raw material for aluminium production thermal insulator for the top of electrolytic cells coating for prebaked anodes absorbent filter for cell emissions Bauxite mining Aluminium is the third most abundant element in the earth's crust after oxygen and silicon. Alumina production Bauxite is washed, ground and dissolved in caustic soda (sodium hydroxide) at high pressure and temperature. The resulting liquor contains a solution of sodium aluminate and undissolved bauxite residues containing iron, silicon, and titanium. These residues sink gradually to the bottom of the tank and are removed. They are known colloquially as red mud. The clear sodium aluminate solution is pumped into a huge tank called a precipitator. Fine particles of alumina are added to seed the precipitation of pure alumina particles as the liquor cools. The particles sink to the bottom of the tank, are removed, and are then passed through a rotary or fluidised calciner at 1100°C to drive off the chemically combined water. The result is a white powder, pure alumina. The caustic soda is returned to the start of the process and used again. Technologies There are two main types of aluminium smelting technology - Söderberg and Pre-bake. The principal difference between the two is the type of anode used. Söderberg Cell: Söderberg technology uses a continuous anode which is delivered to the cell (pot) in the form of a paste, and which bakes in the cell itself. Prebake Cell: Pre-bake technology uses multiple anodes in each cell, which are pre-baked in a separate facility and attached to rods that suspend the anodes in the cell. New anodes are exchanged for spent anodes - anode butts - being recycled into new anodes. The newest primary aluminium production facilities use a variant on pre-bake technology called Centre Worked Pre-bake Technology (CWPB). This technology provides uses multiple point feeders and other computerised controls for precise alumina feeding. A key feature of CWPB plants is the enclosed nature of the process. Fugitive emissions from these cells are very low, less than 2% of the generated emissions. The balance of the emissions is collected inside the cell itself and carried away to very efficient scrubbing systems, which remove particulates and gases. Computer technology controls the process down to the finest detail, which means that occurrence of the anode effect - the condition that causes small quantities of perfluorocarbons (PFCs) to be produced - can be minimised. All new plants and most plant expansions are based on pre-bake technology. Processing Aluminium is such a versatile metal, it can be processed in many different ways depending on the application required. Aluminium can be alloyed with other materials to make an array of metals with different properties. The main alloying ingredients are iron, silicon, zinc, copper and magnesium. Cast Aluminium can be cast into an infinite variety of shapes. The statue of Eros in London's Piccadilly Circus erected in 1893 is cast aluminium. Rolled Aluminium can be rolled into plate, sheets, or wafer thin foils the thickness of a human hair. The rolling process changes the characteristics of the metal, making it less brittle and more ductile. Extruded Aluminium can be extruded to form intricate shapes and sections. It is heated to around 500ÂșC and pushed through a die at great pressure. Forged Aluminium can be forged by hammering to make stress-bearing parts for aircraft and internal combustion engines. Joined, formed and milled Aluminium can be joined together by welding, adhesive bonding, riveting or screwing. It can be formed or shaped by bending or superplastic moulding. It can also be milled or turned on a lathe. Heat treatment The properties of the metal can be modified through heat treatment or mechanical working. Anodised The appearance can be modified by surface treatments such as anodising or powder coating. Fine aluminium products Aluminium powder, flake and paste can be formed by blowing gas under pressure at molten aluminium. This process forms droplets of different sizes. These aluminium products are used in explosives, rocket fuel, metallurgy, chemicals, inks, and decorative materials. Chemicals Aluminium chemicals are important in water treatment, papermaking, fire retardants, fillers and pharmaceuticals. Recycling process All aluminium products can be recycled after use. Its recyclability is one of the huge benefits of using aluminium. Scrap must be of appropriate quality before it can be melted down. To obtain this level of quality, all adherent materials must be removed. Depending upon the type of contamination present, some scrap must be processed; for example, beverage cans must have their lacquer removed prior to remelting. Once sorted, scrap aluminium is then loaded into a furnace, which melts the aluminium completely. This molten metal is then cast or processed - using the same techniques as primary processing. All aluminium that is recycled is described as either new scrap or old scrap. New scrap New scrap is that surplus material that arises during the manufacture and fabrication of aluminium alloys up to the point where they are sold to the final consumer. Examples include the trimmings from the edges of sheet aluminium, turnings and millings from aluminium fabrication and surplus extrusion discards. As such new scrap tends to come from the manufacturing industry, is of a known quality and composition and can be processed with very little preparation. Old scrap Old scrap is material that has been used by the consumer and subsequently discarded. This can include a wide range of items such as used beverage cans, car cylinder heads, window frames or electrical cabling. Value of scrap Anything made of aluminium can be recycled repeatedly not only cans, but plates and pie moulds, window frames, garden furniture and automotive components are melted down and used to make new products. The recycling of aluminium requires only 5% of the energy to produce recycled metal as compared to primary metal and generates only 5% of the green house gas emissions. Scrap aluminium therefore has significant value and commands good market prices. So much so that, aluminium companies have invested in dedicated state of the art processing plants to recycle aluminium. In the case of beverage cans, the process uses gas collected from burning off the coating to preheat the material prior to processing. By recycling aluminium products we: eliminate waste save energy conserve natural resources reduce the use of city landfills provide added revenue for recyclers Isolation Isolation: aluminium would not mormally be made in the laboratory as it is so readily available commercially. Aluminium is mined in huge scales as bauxite (typically Al2O3.2H2O). Bauxite contains Fe2O3, SiO2, and other impurities. In order to isolate pure aluminium, these impurities must be removed from the bauxite. This is done by the Bayer process. This involves treatment with sodium hydroxide (NaOH) solution, which results in a solution of sodium aluminate and sodium silicate. The iron remains behind as a solid. When CO2 is blown through the resulting solution, the sodium silicate stays in solution while the aluminium is precipitated out as aluminium hydroxide. The hydroxide can be filtered off, washed, and heated to form pure alumina, Al2O3. The next stage is formation of pure aluminium. This is obtained from the pure Al2O3 by an electrolytic method. Electrolysis is necessary as aluminium is so electropositive. It seems these days that electrolysis of the hot oxide in a carbon lined steel cell acting as the cathode with carbon anodes is most common. Uses of Aluminum Aluminum metal is very useful for packaging. It is used in cans and aluminum foil. Aluminum borohydride is added to jet fuel. Electrical wiring is sometimes made from aluminum or a combination of aluminum and copper. Lots of normal household items are made out of aluminum. Cutlery, cooking utensils, baseball bats and watches often are often made out of aluminum. Hydrogen gas, an important fuel in rockets, can be obtained by reacting aluminum with hydrochloric acid. Super purity aluminum (from 99.980 to 99.999% pure aluminum) is used in electronic equipment and CDs. Many car, airplane, truck, train, boat and bicycle parts are made from Al. Some countries have coins that are made from aluminum or a combination (alloy) of copper and aluminum. Aluminum is great at absorbing heat energy. Therefore, it is used in electronics (eg. computers) and transistors as a heat sink to prevent overheating. Believe it or not, street lights and sailing ship masts are both made from aluminum. Aluminum borate is used in the manufacture of glass and ceramics. Other compounds of aluminum are used in antacid tablets, water purification, the manufacture of paper, paint manufacturing and the manufacture of synthetic gemstones. Aluminum: Uses of Aluminum Aluminum is a silvery-white and a very popular metal that has many uses and useful properties. No other metal has as many uses as aluminum does. As this metal has recycling properties, it is enough to meet all our daily needs. It is used at home, in construction, in several car parts and also in most of the modes of transportation. It is quite surprising to see that there are so many uses of the metal. Where can you find more information? Using all of the information that I have collected and presented throughout this project I will answer some key questions about aluminum. Over the course of my investigation into Aluminum I have used these questions to channel my lines of enquiry to provide the most thorough answers possible, and to give my research a focus. How does aluminum avoid corrosion? Aluminum forms a 1 nm (or sometimes slightly thicker) layer of aluminum oxide on its surface when it is exposed to air. Even though aluminum is reactive this oxide layer prevents the actual aluminum making contact with other elements which it could react with. In this way aluminum avoids reaction and corrosion. This is a particularly important feature for aluminum is its outdoor uses where being resistant to weathering is essential. If the layer breaks it immediately reforms when the aluminum is next in contact with oxygen. Why is aluminum used so much in transport? Aluminum is a low density material which gives it its lightweight property. When mixed with other elements in alloys it is also very strong. This unique combination of strength and weight is essential for the transport industry. As Force = Mass x Acceleration, the bigger the mass the more force will be needed to achieve a certain acceleration. This means that using aluminum is transport vessels saves money on fuel, allows more cargo to be carried, and is better for the environment. Aluminum's corrosion resistance perfect this profile making it absolutely ideal for its uses in aeroplanes, trains, cars, and ships. Why is aluminum so reflective? Aluminum can reflect up to 97% of light that falls upon it when highly polished, but how does it do this? Reflections occur when light hitting a material provides enough energy for an electron to move to an excited state, from where it emits a photon of light upon returning to its ground state. In aluminum there are many electrons that are free to easily become "excited" like this, this makes it an extremely good reflector. Why is aluminum not magnetic? In all materials electrons orbit atoms, and as each of the electrons has an electric charge each of these electrons produces a tiny magnetic field. In most materials electrons pair up in energy sublevels with opposite "spin" properties, so the effect of their tiny magnetic field is cancelled out by each other. However in some materials the electrons frequently don't have to pair up so an overall magnetic field can be created if a magnet forces the electrons to align in the same direction which it does in metals like iron. In iron and steel some electrons stay aligned, and this is why large magnets are often made out of these materials. In aluminum the electrons don't give an overall magnetic field in one direction, so it isn't magnetic. |
|
What's aluminum like? What's aluminum used for? How is aluminum made? |
|
Aluminum Coil Aluminum Plate Aluminum Sheet Aluminum Sign Blanks Aluminum Extrusions 



|
| Aluminum/Brass Faucets |
|
Heat sinks for electronic appliances such as transistors and CPUs.
Powdered aluminium is used in paint, and in pyrotechnics such as solid rocket fuels and thermite. |
| Aluminium can be reacted with hydrochloric acid or with sodium hydroxide to produce hydrogen gas. |
| http://www.aluminum.org |
